Featured
- Get link
- X
- Other Apps
Dissolving Sugar In Water Physical Or Chemical Change
Dissolving Sugar In Water Physical Or Chemical Change. Is sugar dissolving a physical change? This is a physical change, because the sugar retains its chemical structure, and when you boil off the water or.
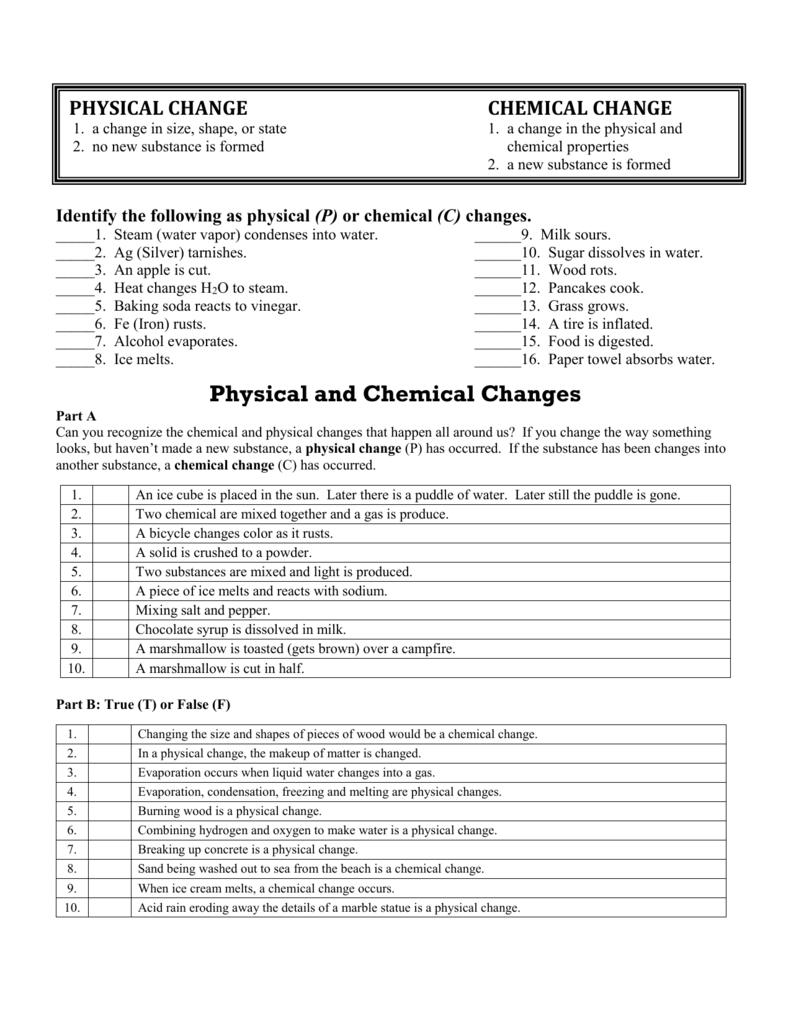
The solvent is the one doing the dissolving water. Therefore, this is a chemical change. Is dissolving of sugar in water a chemical or physical change?dissolving sugar in water is a physical change.
Dissolving Sugar In Water Is An Example Of A Physical Change.
If the chemical formulas do not change, then any changes are physical changes. Dissolving the sugar in the water makes a homogenous mixture and once dissolved a solution is made by the combination of a solute (sugar) and solvent (water). In order for sugar in water to be a.
Students Can Find A Detailed Difference Between Physical And Chemical Change On Vedantu.
A chemical change produces new chemical products. Sugar dissolving in water is a physical change. The sugar crystals of sugar breaks down in the molecules and remain suspended in the water.
Q1Classify The Changes Involved In The Following Processes As Physical Or Chemical Changes.
The reactant (sodium chloride, or nacl) is different from the products (sodium cation and chlorine anion). Dissolving sugar in water is an example of a physical change. You can tell this because if you were to dissol.
So Option B Is A Correct Option.
The chemical formulas at the end are the same as they were at the start, so this is a physical change. What change is melting sugar? In order for sugar in water to be a chemical change, something new would need to result.
In Contrast, Dissolving A Covalent Compound Like Sugar Does.
A chemical reaction would have to occur. It is because no new substance is formed. Thus, any ionic compound that is soluble in water would experience a chemical change.
Popular Posts
The Bar-Kays You Made A Change In My Life
- Get link
- X
- Other Apps
Comments
Post a Comment